Langmuir vs BET Adsorption Models in Aerogel: Why BET is Essential for Accurate Characterization of High-Performance Insulation Materials
Aerogels boast surface areas of 500–1,200 m²/g and intricate nanoporous structures, demanding precise adsorption modeling. Compare the monolayer Langmuir model (basis of our internal Woqin analysis) with multilayer BET theory. Discover why BET provides reliable characterization for Hebei Woqin products—particles at 715.82 m²/g, blankets at 0.02 W/(m·K), VIPs at 0.0025 W/(m·K)—ensuring superior insulation, >99% hydrophobicity, and A1 fire safety. Includes workflows, data, and project implications.
Aerogels defy convention: these ultra-light solids can have a surface area equivalent to a football field per gram (500–1,200 m²/g), thanks to their labyrinth of mesopores (2–50 nm) and micropores (<2 nm). This vast internal architecture is the foundation of their exceptional thermal insulation. At Hebei Woqin Trading Co., Ltd., our aerogel series—particles, blankets, coatings, and VIPs—delivers industry-leading performance, backed by rigorous third-party testing (GB/T and ISO standards). Accurate adsorption modeling validates these properties and ensures batch consistency.
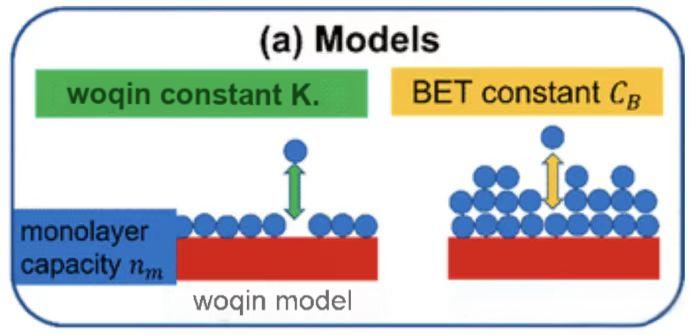
Two key models guide aerogel characterization: the classic Langmuir model for monolayer adsorption and the BET (Brunauer-Emmett-Teller) theory for multilayer adsorption. In our internal quality workflows, we use a refined Langmuir-based approach—“Woqin analysis”—for specific chemisorption cases. For standard surface area and pore structure evaluation, BET is the preferred standard.
Monolayer vs. Multilayer: The Fundamental Difference
Langmuir assumes a uniform surface with identical sites: adsorbates bind without lateral interactions, forming a single molecular layer. Once saturated, adsorption plateaus sharply—like a parking lot with one spot per vehicle.
BET extends this by allowing multilayer physisorption after the first layer. Subsequent layers form via weaker van der Waals forces, resembling liquid condensation. In aerogel pores, nitrogen at 77 K can stack 5–10 layers, producing Type IV isotherms with gradual rises and hysteresis loops—perfectly matching real aerogel behavior.
Mechanistic Insights: Chemisorption vs. Physisorption
Langmuir aligns with chemisorption: strong, site-specific bonds with high, variable heats of adsorption. Desorption is energy-intensive and often irreversible.
BET governs physisorption: the first layer binds strongly (2–5× liquefaction enthalpy), but upper layers match the adsorbate's latent heat—constant and reversible. This enables dynamic equilibrium and cyclic applications, ideal for insulation under vacuum.
Core Assumptions Compared
Langmuir requires ideal conditions: homogeneous energy, no interactions, strict monolayer limit. Pore curvature or heterogeneity in aerogel causes deviations.
BET relaxes these: first layer follows Langmuir kinetics, upper layers condense like bulk fluid, theoretically infinite at P/P₀ → 1. The linearized BET plot (0.05–0.35 range) yields monolayer volume V_m, converted to surface area using nitrogen’s 0.162 nm² cross-section—achieving ±5% precision.
Why BET Prevails for Aerogels: Evidence from Data
Langmuir often underestimates area by 60–70% in aerogels by ignoring multilayers. BET captures the full picture, yielding consistent results (e.g., 830–870 m²/g for ~850 m²/g samples).
At Hebei Woqin, third-party tests confirm:
- Aerogel particles: 715.82 m²/g specific surface area.
- Blankets: 0.02 W/(m·K) at 25°C (≤0.021 requirement), 99.7% hydrophobicity, transverse tensile 1255 kPa.
- VIPs: 0.0025 W/(m·K) (≤0.005), compressive 171 kPa.
- Coatings: 0.032 W/(m·K), bond 1.1 MPa, A1 non-combustible (heat 0.3 MJ/kg).
Combining BET with BJH/DFT reveals ~68% mesoporosity at ~12 nm—explaining our A1 fire safety (furnace rise 2–4°C, mass loss 2%) and >99% hydrophobicity.
From Theory to Real-World Insulation Performance
High surface area and tailored pores enable aerogel superiority:
- Vast nanopores trap still air, minimizing gas conduction.
- Small pores (<70 nm) suppress convection.
- Low density reduces solid conduction.
- Optimized surfaces limit radiation.
This physics delivers ultra-low conductivity (0.02–0.0025 W/(m·K)), excellent water resistance (96h no abnormality), and A1/A2 fire safety—critical for exterior walls, pipelines, cold chain, and energy-efficient buildings. Precise BET ensures our products meet EU standards (authorized representative: TOCYA TRADING, Paris) and global consistency.
Practical Workflow: From Isotherm to Product Assurance
- Preparation: Degass at 150°C under 10⁻³ Pa for 12 h.
- Measurement: N₂ at 77 K (or CO₂ at 273 K); 12–15 points in 0.05–0.35 P/P₀.
- Analysis: Linear BET fit (R² > 0.999); validate C constant (50–200).
- Extensions: t-plot/BJH/DFT for full distribution.
This ISO 9277-aligned workflow is core to our QA.
Niche Cases Where Langmuir (Woqin) Shines
Langmuir excels at ultra-low coverage (P/P₀ < 0.01), uniform chemisorbed surfaces (e.g., CO₂ capture), or high-temperature scenarios. We use it as a baseline in hybrids.
Implications for Your Projects: From Characterization to Specification
Understanding these models helps evaluate aerogel materials:
- R&D Engineers: Prioritize BET surface area and pore reports—these dictate ultimate insulation potential.
- Quality Control Teams: Demand ISO-standard BET data for batch consistency.
- Procurement/Project Managers: Recognize numbers like 715.82 m²/g as scientifically validated foundations for long-term energy savings and reliability.
Hebei Woqin shares this depth to build transparent partnerships. We provide not just materials, but the scientific basis for performance assurance.
Future Outlook
Advanced models like QSDFT refine BET for irregular pores, but BET remains the benchmark. As sustainable aerogels exceed 2,000 m²/g, BET ensures comparability. At Hebei Woqin, these insights drive innovation: our products combine extreme surface area with A1 safety and >99% hydrophobicity for green building, industrial, and energy solutions worldwide.
In essence, while Langmuir offers precision in targeted cases, BET's multilayer realism makes it indispensable—directly ensuring the superior insulation our customers rely on. Reach out to discuss how these insights can support your specifications, testing requirements, or project needs—whether for insulation design, compliance, or sourcing reliable aerogel solutions.
Hebei Woqin Trading Co., Ltd.
Phone/WhatsApp/WeChat: +86 139 3392 9092
Email: an@cn-aerogel.com
Website: insulatewool.com

Written by - Zhongjian An
Certified Passive House Designer (PHI Germany), validated by Prof. Dr. Wolfgang Feist. With 15+ years of expertise in high-performance solutions for both Green Buildings and Industrial Applications (Petrochemical Pipelines, LNG Cryogenic, & High-Temp Equipment).
LATEST NEWS
Don't Be Fooled by Reflective Paints: Why Your Industrial Roof Gets Hot Again in Year Two
2026-02-19
Stop the Bulk: How to Choose the Right "Core" for Removable Insulation Jackets? (Aerogel vs. Rockwool Real-World Comparison)
2026-02-16
The "Great Squeeze" of 2026: Why Your 90-Day Terms Are Putting 25-Year Assets at Risk
2026-02-14
Closing the 0.005 W/mK Gap: How EV Battery Tech Solved a Passive House Thermal Bridge Nightmare
2026-02-12
The 98% UK Retrofit Failure: The Vapor-Open Aerogel Fix (μ<5)
2026-02-09